
As we pass the halfway mark of 2025, several high-profile product liability litigations are gaining traction in courts across the country. From pharmaceuticals to social media platforms and even food manufacturers, companies are facing complex claims related to failure to warn, product defects, and long-term health consequences.
For mass tort attorneys—whether plaintiff or defense—as well as in-house counsel and insurers, keeping a close eye on these cases is critical for risk assessment and strategy development.
At the start of the year, we highlighted five product liability cases to watch. Below is a status update on each, including current filings, procedural developments, and the core allegations.
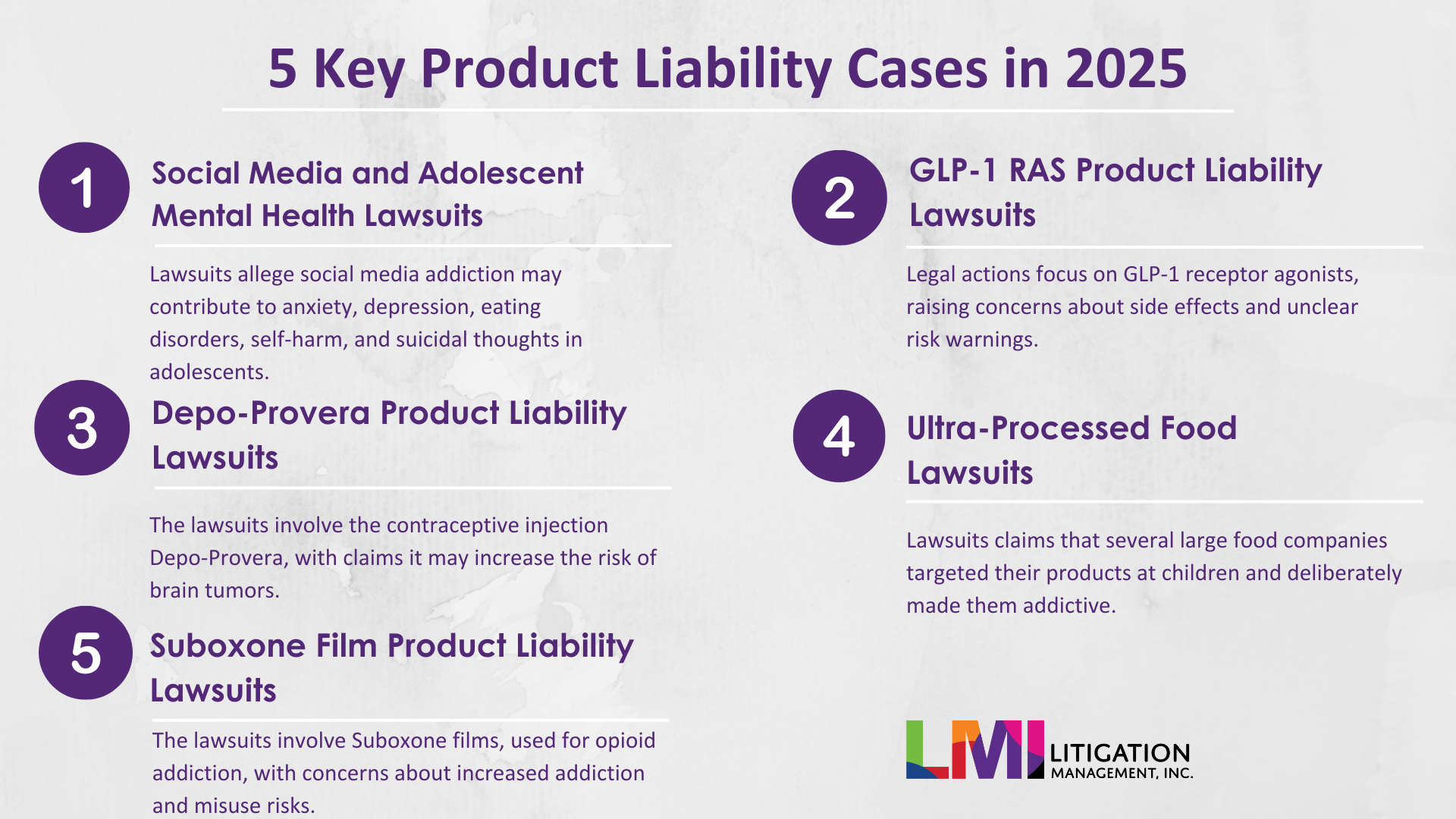
Where the Top 5 Product Liability Lawsuits Stand Mid-Year 2025

1. Social Media and Adolescent Mental Health Lawsuits
Allegations and Parties Involved:
This MDL consolidates lawsuits brought by individual minors, parents, and school districts alleging that major social media platforms, including Meta (Facebook, Instagram), TikTok, Snap Inc., and YouTube, designed their products to be addictive and failed to warn users about the risks to adolescent mental health. Plaintiffs claim these platforms contributed to rising rates of anxiety, depression, self-harm, and suicide among teens.
Federal and State Updates:
MDL No. 3047 – IN RE: Social Media Adolescent Addiction/Personal Injury Products Liability Litigation continues to grow rapidly, with 1,997 actions pending as of July 1, 2025.
In a pivotal June 16 Case Management Order, Judge Yvonne Gonzalez Rogers selected six school district cases and five individual plaintiffs’ cases as bellwether trials, a major step toward shaping potential settlements and understanding case themes.
Parallel state proceedings are consolidated in California under JCCP Re: Social Media Adolescent Addiction/Personal Injury Products Liability, signaling a growing multijurisdictional challenge for defendants.
How Attorneys Can Prepare for the Next Phase of the Case
- School system claims may require unique data gathering from institutional sources. Prepare for high-volume document and record requests.
- Psychological evaluations and educational records will be central to causation arguments. Establish medical record review protocols now.
- Implement legal tech platforms to manage large datasets. Identify patterns in claims and support analytics-driven strategy.
- Partner with a litigation support provider to ensure secure handling of sensitive data, especially for minor plaintiffs.

2. GLP-1 Receptor Agonists (Ozempic/Wegovy) Lawsuits
Allegations and Parties Involved:
Plaintiffs in this litigation claim that GLP-1 medications, primarily marketed for diabetes and weight loss under brand names like Ozempic, Wegovy, and Mounjaro, are associated with serious, undisclosed side effects.
Alleged injuries include gastrointestinal issues, gallbladder disease, and, more recently, vision loss from NAION (nonarteritic anterior ischemic optic neuropathy).
The primary defendants include Novo Nordisk and Eli Lilly, among others, involved in manufacturing or distributing these medications.
Federal and State Updates:
In MDL No. 3094 – IN RE: Glucagon-like Peptide-1 Receptor Agonists Products Liability Litigation, the number of pending actions has climbed to 2,040 as of July 1, 2025.
In June, 21 plaintiffs petitioned for a Multicounty Litigation (MCL) in New Jersey state court specific to NAION-related injuries, suggesting a potential expansion in the scope of the litigation and new challenges in causation and medical proof.
How Attorneys Can Prepare for the Next Phase of the Case
- Prepare to collect and review ophthalmology and neurology records related to vision loss and rare complications.
- Standardize medical record review processes to efficiently screen and qualify large volumes of claims.
- Use analytics tools to track side effect patterns across demographics and medications.
- Collaborate with a litigation support partner who specializes in medical record review to identify medically complex claims and prioritize them for deeper analysis.

3. Depo-Provera Product Liability Lawsuits
Allegations and Parties Involved:
This litigation centers on claims that Depo-Provera, a long-acting injectable contraceptive manufactured by Pfizer, causes significant and permanent side effects such as bone density loss, hormonal imbalances, and mental health disturbances.
Plaintiffs argue that the manufacturer failed to adequately warn users of these risks.
Federal and State Updates:
MDL No. 3140 – IN RE: Depo-Provera Products Liability Litigation now includes 435 actions pending as of July 1, 2025.
A motion for Judicial Council Coordination Proceeding (JCCP) is currently pending in California to consolidate related state court actions. In the Philadelphia Court of Common Pleas, it appeared a coordinated mass tort was taking shape until a judge severed nearly all 100 plaintiffs from the consolidated case and allowed them to refile individually. As a result, Pfizer withdrew its petition for coordination.
A major legal turning point is approaching on September 29, when Judge M. Casey Rodgers will hear Pfizer’s motion for federal preemption, a motion that, if granted, could significantly narrow the scope of the litigation.
How Attorneys Can Prepare for the Next Phase of the Case
- Gather comprehensive long-term medical records to support or challenge causation related to extended product use.
- Create systems to track hormone, bone, and reproductive health outcomes across claims.
- Stay alert for jurisdictional developments as state-level coordination may impact how records are collected and shared.
- Consider outsourcing data analytics and medical chronology development to support scientific arguments around causation.

4. Ultra-Processed Food Lawsuits
Allegations and Parties Involved:
In an evolving area of product liability litigation, plaintiffs are suing manufacturers of ultra-processed foods, such as snack and convenience food companies, alleging that these products contribute to chronic disease, obesity, and other health problems, and that companies misrepresented or concealed the health risks associated with prolonged consumption.
Defendants in these cases include major food and beverage corporations that produce packaged snacks, processed meals, and sugary beverages.
Federal Updates:
Still in early procedural stages, this litigation is closely watched for its potential to reshape liability frameworks in the food industry.
The defendants’ motion to dismiss is scheduled for argument on August 1, 2025. A denial of the motion could pave the way for discovery and potentially open the door to more widespread claims.
How Attorneys Can Prepare for the Next Phase of the Case
- Begin compiling health records, food consumption data, and lifestyle documentation that could support or challenge claims of harm.
- Explore partnerships with medical record review companies that specialize in complex liability cases to assess the strength of alleged causal links between processed foods and chronic disease.
- Use AI-driven data management tools to handle large and diverse datasets like product usage and medical conditions.
- Be proactive in planning discovery workflows, including early vetting and categorization of claims with litigation support teams.

5. Suboxone Film Product Liability Lawsuits
Allegations and Parties Involved:
This litigation alleges that Suboxone Film, a treatment for opioid dependence, causes tooth decay and dental damage that was not adequately disclosed. Plaintiffs argue that the product’s acidic pH and method of administration (under the tongue or in the cheek) contributed to the injuries. The primary defendant is Indivior, the manufacturer of Suboxone.
Federal Updates:
The court has taken steps to streamline proceedings. In a recent Case Management Order, it authorized the filing of up to 100 plaintiffs in a single complaint, which is expected to accelerate the pace of filings.
Additionally, the court has laid out protocols for medical record collection, depositions, and a bellwether selection process, indicating that the case is moving toward more active discovery and trial preparation.
How Attorneys Can Prepare for the Next Phase of the Case
- Establish dental and oral health records workflows, including dental history, treatment plans, and cost of care.
- Ensure claims processing infrastructure can handle group filings and bulk data intake.
- Coordinate with experts to evaluate preexisting conditions vs. Suboxone-specific injuries.
- Establish case tracking and analytics dashboards to monitor trends and outcomes across bundled complaints.
Strategic Outlook: Preparing for the Next Phase of Product Liability Lawsuits
As each of these product liability cases advances through discovery and bellwether preparation, the second half of 2025 will demand even greater precision from legal teams.
Whether you're on the plaintiff or defense side, or managing litigation risk in-house, now is the time to:
- Standardize data collection and document workflows
- Establish scalable medical record review processes
- Leverage litigation analytics to prioritize and assess cases
- Invest in legal technology that supports end-to-end case management
- Engage experienced litigation support partners who can provide the systems, staff, and strategy to stay ahead
By taking proactive steps now, firms and legal departments can reduce downstream inefficiencies, improve case outcomes, and better manage the growing complexity of mass tort litigation.
Need support navigating large-scale litigation? LMI helps law firms, corporations, and insurers streamline data management, medical analysis, reporting and analytics across complex matters.
Let’s talk about how we can help you prepare for what’s next. Complete our contact form, and we’ll follow up with solutions tailored to your needs.